Howdy! We are back this week with a new topic which is around Clinical Trials and how Decentralized trials are helping accelerate the trial process.
Before we start, let us look at clinical trial as a process, and how the landscape is evolving. As we all know, Clinical trials have largely been in existence as scientific answers to questions like “Does a treatment work?”, “Does it work better than other treatments?”, “Does it have side effects?”, or for providing critical information like “cost-effectiveness of a treatment”, “clinical value of a diagnostic test” and “how a treatment improves quality of life”.
As per researchandmarkets.com –
The U.S. clinical trials market size is expected to reach USD 32.4 billion by 2028, registering a CAGR of 5.3% over the forecast period.
The delivery of new medicines to the marketplace is the lifeblood of the pharmaceutical industry and clinical trials are critical in evaluating the safety and effectiveness of a medical, surgical, or behavioral intervention.
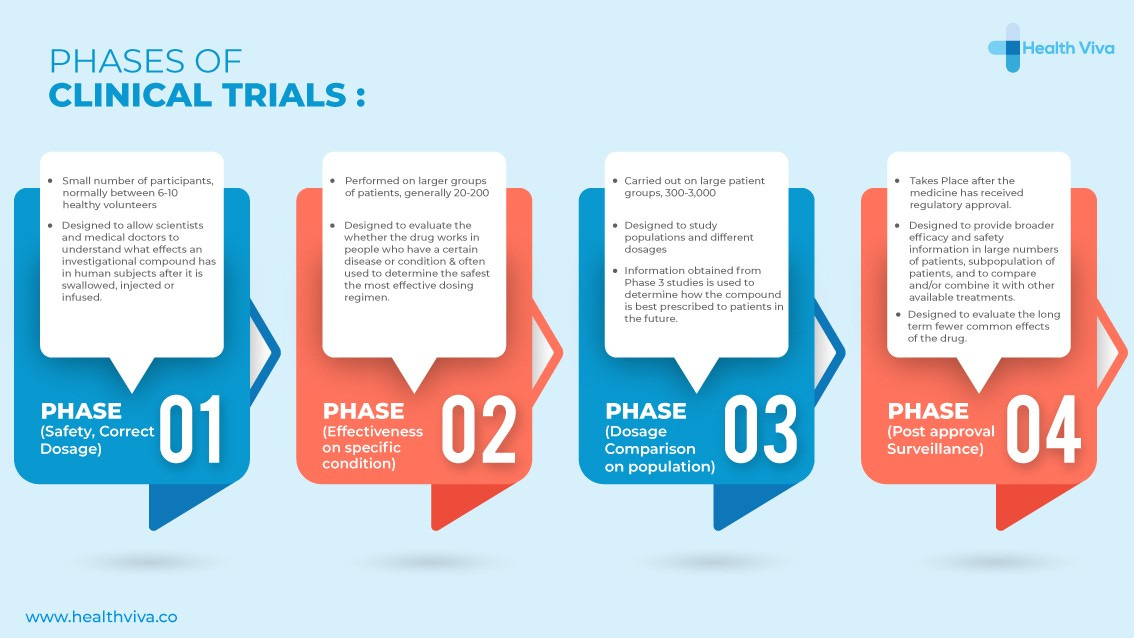
Phases of Clinical Trials
Clinical trials are broken down into four phases categorized as Phase I to IV trials. They are generally described as follows:
Challenges facing Clinical Trials
Clinical trials are not easy – being complex, lengthy, and required to follow several rules and regulations to ensure compliance with different standards.
As per an article published in Mar 2019 at policymed.com -
Developing a new prescription medicine that gains marketing approval is estimated to cost drugmakers $2.6 billion
Some of the common challenges faced by organizations to carry out these complex, expensive trials are as below:
Subject recruitment and retention
Subject (also referred to as Patient) recruitment (recruiting the right subject for the trial) is a huge challenge and around 80% of these studies are either halted or even closed due to low subject recruitment, 69% of them fail pre-screening, 58% decline consent. Extensive paperwork and the amount of travel to and from clinical trial centers have traditionally hindered efforts of recruitment. More recently, fears of attending appointments in a healthcare setting, where there is a perceived risk of being infected by COVID-19, has provided a huge setback.
Compliance with several rules and regulations
Since trials are full of complex activities with involvement of human subjects, untested drugs, devices, and procedures, ensuring compliance becomes mandatory to ensure safety as well as maintaining ethics of the trials. This results in the process being time-consuming which often create a bottleneck.
Managing multiple sites
Clinical trials usually occur over multiple sites which involve diverse subjects, more vendors, procedures, diverse compliance requirements, and coordination efforts, leading to more complexities down the line.
Preventing duplicate subjects
Subjects tend to sign up for multiple trials either simultaneously or consecutively, which may lead to skewing overall data leading to data bias. This may also cause billions of dollars in losses or delays in approvals from pharmaceutical regulatory agencies.
Therefore, future clinical trials need to factor in these bottlenecks in the pursuit of patient centric and robust trial conduct.
Decentralization of trials- Paving the way for the future
“Patient-centricity” is the buzzword today and it is time to rethink the concept of traditional
clinical trial model and refresh how research studies are conducted, while keeping the patient at the heart of this emerging paradigm.
Decentralization of trials is a novel concept and is an attempt to look for areas where technology powered solutions can provide an alternative to a site-anchored, inflexible system that can eventually reduce the time and financial burden on subjects, as well as accelerate recruitment and reduce drop-out rates.
Decentralized clinical trials (DCT) can be defined as studies “executed through the use of technology using processes differing from the traditional clinical trial model.”
As per market report –
Global Decentralized Clinical Trials Market was valued at US$7,235.5 million in 2021 and is projected to grow at a CAGR of 6.95% By 2032:
- By Visiongain Research Inc
Decentralized clinical trials use technology to monitor subjects and collect real-time data and can reduce study costs and help get medications to market more quickly.
There are different ways to describe the concept of decentralized trials, including virtual, home, remote, and site-less. Irrespective of the phrases used to define DCT, the goal is the same: making clinical trials easier and more effective.
DCTs have quickly become a strategic priority, that has recently skyrocketed with the advent of the Covid-19 pandemic and is expected to endure long after the COVID-19 pandemic ends.
Some of the highlights of DCT are as below:
· Decentralized trials offer a patient-centric approach, addressing various patient needs that often go unmet in traditional designs. It enables building convenience and flexibility into the process as the patient can undertake the trial from the comfort of home.
· DCT have been largely enabled by advancement of technologies which have shown to impact key trial-related activities: communication, enrollment, recruitment, consent, and continuous data acquisition.
· A constellation of evolving technologies and services such as Electronic Patient Consent, Telehealthcare, Remote Patient Monitoring, and Electronic Clinical-outcome Assessments (eCOAs) have allowed to maintain links to trial participants without in-person visits making clinical trial participation easier for patients. This in turn can speed up recruitment, increasing compliance and reducing drop-out rates.
· Using the technology of Electronic Consent there may be minimal need for travel even for the initial paperwork. This leads to more adherence to compliance and protocols and in turn leading to quicker, more accurate results.
· Mobile and home healthcare technologies enable more procedures to occur away from research sites.
· With DCT, diverse population can be accessed for the trials since it is no longer grounded to a specific healthcare facility. This diversification is crucial in helping ensure the safety and efficacy of treatments across the entire population.
· With the advent of the DCT it should not be misconstrued that the trials will be entirely virtual. Fully virtual trials will remain limited to a narrow set of use cases, such as a well-characterized drug with few adverse events in a mild indication, with end points suited to remote measurement.
· Traditional sites will still be the solution for complex procedures and specialized assessments, such as screenings and magnetic resonance imaging.
While there are significant benefits to using DCTs, they are relatively new, and organizations need to develop best practices to ensure trial quality and data privacy.
Covid-19 driven adoption of technologies such as Telemedicine, Remote Patient Monitoring, along with AI and Big Data have shown potential to address key gaps in today’s clinical trial landscape. With further advancements in these technologies, future trials could be reshaped to provide alternative methods to traditional study design practices.
Industry News: Let us look at some of the industry happenings around Decentralized trials -
Medable Launches Partner Network to Accelerate Innovation and simplify deployment of Decentralized Clinical Trials
Parexel Extends Strategic Partnership with Medidata to Enhance Delivery of Decentralized Clinical Trials
Medidata Expands and Strengthens Decentralized Clinical Trial (DCT) Capabilities Through Groundbreaking Partnership With Circuit Clinical
YPrime Acquires Tryl, a Patient Engagement Solutions Company, Adding More Capabilities to Support Decentralized Clinical Trials
Philips Introduces First At-Home, 12-Lead ECG Integrated Solution for Decentralized Clinical Trials
Seqster Partners With United BioSource to Enable Decentralized Clinical Trials
HumanFirst Opens Up Atlas Platform to Scale Use of Decentralized Clinical Trials
As we can conclude, investments are on a rise around DCT, and DCT is going to remain . If you are working on leveraging Decentralized Trials, please feel free to share your story. You can reach us at healthviva@taliun.com
Adios!
Team HealthViva